http://uefiscdi.gov.ro/Project financed by UEFISCDI, Executive Agency for Higher Education, Research, Development and Innovation Funding Human Resources PROGRAMME, Research projects for the stimulation of the funding of young independent research teams (TE)
Host institution: Politehnica University Timişoara
Project PN-II-RU-TE-2012-3-0198
Contract number: 20/26.04.2013
Project title: New approach of using ionic liquids (ILs) as "green extractants" in the adsorption process of radionuclides from waste aqueous solutions
The purpose of the site is to present the progress and the results achieved within the research project no. 3-0198, PN-II-RU-TE-2012 competition. The project is financed by the Executive Agency for Higher Education, Research, Development and Innovation Funding UEFISCDI. The registration number of the project financing contract is 20/26.04.2013
The host institution is Universitatea Politehnica Timisoara.
Project summary:
The overall goal of the proposed project is to investigate a new approach of using the room temperature ionic liquid (RTIL) as extractants impregnated onto various solid supports in the adsorption process of radionuclides from waste aqueous solutions. The project has an interdisciplinary character presenting an integrated concept of waters depollution with radionuclides content. The use of ionic liquids (ILs) so called “green extractants”, instead of the volatile organic solvents as new separation media of metals from aqueous solutions is in agreement with the principle of the sustainable development and in full compatibility with the environment protection. Various ILs will be used, which will be impregnated onto various solid supports, and the resulted extractant impregnated materials (EIM) after characterization, will be used as adsorbent materials in the removal process of radionuclides from waste aqueous solutions. Using some solids supports impregnated with ILs as adsorbent materials is expected to achieve very good performance in the removal process of radionuclides from waste aqueous solutions because the adsorbent properties of the solid supports and the advantageous properties of ILs are combined, thus opening and establishing the new science based on both adsorption technology and ionic liquids. The benefit of the proposed research is provided by the scientific establishing and understanding of the mechanism of radionuclides adsorption from waste aqueous solutions
The implementation team
Project leader: S.l. Dr. Ing. Lavinia Lupa
Researcher: Prof. Dr. Ing. Petru Negrea
Researcher: Conf. Dr. Ing. Adina Negrea
Researcher: S.l. Dr. Ing. Mihaela Ciopec
Researcher: S.l. Dr. Ing. Raluca Voda
Researcher: Stud. Ing. Alexandra Bogin
Research report (2013-2016)
Objective 1.1. Impregnation of different solid support with various ionic liquids
The development of ionic liquid-impregnated supports is a link between solvent extraction and advantages of the solid supports, therefore such application lie in the increased selectivity, higher degree of adsorbent-adsorbate interaction, mechanical stability of the solid support. The most relevant criteria for the selection of the support system are: the porosity of the support and the surface properties. A lot of researchers use as solid support the macro porous organic polymers, due to their high surface area and good mechanical stability, suitable for the removal of toxic elements from dilute solution, due to their faster kinetics, ease of regeneration and high adsorption capacity. Others researchers found that among the organic supports the inorganic types have several superior qualities such as: higher thermal and chemical stabilities, well-ordered periodic pore structure, and controllable pore diameter, and particularly in liquid radioactive waste treatment: radiation stability and great selectivity for certain radiological important species. Therefore the adsorption ability of various solid supports impregnated with IL in the removal process of different radionuclides (Cd, Pb, Co, Ni, Cr, Cs, Sr, Tl and La) from aqueous solutions, was studied. It was presented a comparison between the characteristic of the three most used organic materials (Dowex resin, Amberlite XAD7 resin and Crown ether – dibenzo-18-Crown-6) and two inorganic materials (Florisil and Silica), in order to be used as solid supports. For impregnation of the studied solid supports with the IL, Cyphos IL-101 (trihexyltetradecylphosphonium chloride), was used. The obtained impregnated ionic liquids were characterized by Fourier transform infrared spectroscopy FTIR, energy dispersive X-ray analysis (EDX), scanning electron microscopy (SEM). The adsorption capacities of the studied solid supports in the removal process of various radionuclides from aqueous solutions were determined using 2 and 24 hours of contact between the adsorbate and adsorbent.
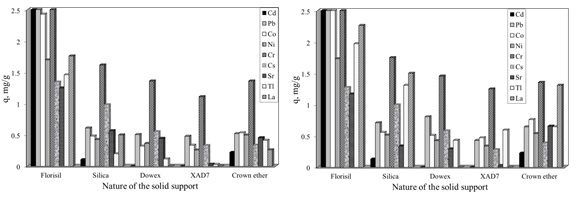
The obtained maximum capacities of the studied solid supports, in the removal process of various radionuclides from aqueous solutions, obtained experimental and obtained from Langmuir plot are presented in next table.
The inorganic solid support increase the adsorption performance of the obtained materials through the impregnation with various ionic liquids, compared with the organic solid supports. Were made detailed studies regarding the removal of Cs+, Sr2+ from aqueous solutions using as adsorbent material Florisil and Silica impregnated with Cyphos IL-101.
|
Parameter |
Radionuclid |
||||||
|
Cs+ |
Sr2+ |
La3+ |
Cr3+ |
||||
|
Florisil |
|||||||
|
R2 qe (exp), mg/g qe (calc), mg/g KL, L/mg |
0.9989 3.39 3.75 0.1175 |
0.9849 2.18 2.37 0.0924 |
0.9872 6.75 7.63 0.0915 |
0.9953 2.68 2.72 0.2808 |
|||
|
Silica |
|||||||
|
R2 qe (exp), mg/g qe (calc), mg/g KL, L/mg |
0.9974 1.69 1.80 0.1415 |
0.9836 1.87 2.29 0.2140 |
0.9922 4.47 5.17 0.0680 |
0.9914 2.50 2.68 0.1285 |
|||
|
Crown ether |
|||||||
|
R2 qe (exp), mg/g qe (calculat), mg/g KL, L/mg |
0.9329 0.55 0.71 0.0983 |
0.9761 1.09 1.64 0.0470 |
0.9906 3.25 4.28 0.0957 |
0.9873 0.90 1.17 0.0748 |
|||
|
Dowex |
|||||||
|
R2 qe (exp), mg/g qe (calc), mg/g KL, L/mg |
0.9966 0.98 1.18 0.1148 |
0.9840 1.45 2.12 0.055 |
- - - - |
- - - - |
|||
A detailed study was realized for the removal process of Sr2+ and Tl+ removal using as adsorbent material an organic solid support styrene-divinylbenzene grafted with aminoethylaminomethyl, which was impregnated with various ionic liquids. Comparing the results obtained for Sr2+ through adsorption using the same ionic liquid for the impregnation was also observed that the inorganic solid support develop higher adsorption capacity than the organic solid support.
Activity 1.1.1 Studiing of various method of impregnation
Because the impregnation method can affect the property and applicability of the obtained material was studied for method of impregnation:
- Ultrasound method;
- Dynamic column method;
- Stirring method;
- Dry method.
The influence of the method of impregnation was determined for the process of Cs+ removal from aqueous solutions using Florisil and Silica impregnated with Cyphos IL-101 (tetradecyl(trihexil)phosphonium chloride). The materials obtained through impregnation of the studied solid support with the studied ionic liquids using various method of impregnation were characterized by Fourier transform infrared spectroscopy FTIR, energy dispersive X-ray analysis (EDX), scanning electron microscopy (SEM) and BET – accelerated surface area analyses. From the characterization of the obtained materials was observed that the IL was bounded on the surface of the studied inorganic supports and the most abounded particles of the IL bounded by the inorganic supports is shown in the case of the impregnation through ultrasound method and through dynamic column method. The studied materials were used in the removal process of Cs+ ions from aqueous solutions containing various concentrations. The results were fitted with the Langmuir isotherm model. Can be observed, that in case of the Florisil impregnated samples are obtained higher adsorption capacities, than in case of the Silica impregnated samples. One can be noticed that in the case of the samples obtained through ultrasound and dynamic columns methods developed higher adsorption capacities than the other samples, results which are in accordance with the conclusions raised from the characterization of the obtained impregnated samples. The ultrasound method of impregnation present the advantages that is not time consuming like the other methods, therefore this method of impregnation was used for the further studies.
|
Inorganic support/method of impregnation |
KL, L/mg |
qm, mg/g |
R2 |
|
Florisil: - Ultrasound - Dynamic column method - Stirring method - Dry Method
|
0.140 0.130 0.182 0.123 |
3.50 3.77 3.08 3.08 |
0.9918 0.9674 0.9978 0.9907 |
|
Silica: - Ultrasound - Dynamic column method - Stirring method - Dry Method
|
0.365 0.615 0.422 0.597 |
1.76 1.80 0.83 1.48 |
0.9957 0.9996 0.9998 0.9994 |
Activity 1.1.2. Variation of impregnation conditions
The influence of the work conditions of ultrasonication method upon the adsorption performance of the obtained materials was studied in order to determine the optimum conditions of impregnation which enhance de adsorption properties of the impregnated materials. The influence of work conditions of impregnation was studied for the system of Florisil impregnation with three ionic liquids (Cyphos IL-101, BmimPF6 and Omim BF4). The obtained materials were also characterized and used in the removal process of Cs+ ions from aqueous solutions. It was observed that for all the cases the increasing of the ultrasonication time lead to a patchy conglomeration of the studied IL onto the surface of Florisil support. The most uniform adherence of the IL particle onto the Florisil is obtained in case of 10 minutes of impregnation. Also at this time the adherence is deeper in the particles of the solid support, in this way the leaching of the IL from the solid support surface during the adsorption experiments is avoided. These results are significant in order to obtain reproducibility of the Cs+ adsorption process. Through the amplitude increasing is achieved an easier transmission of the IL particles through the liquid media until reach the cavities of the solid support, the impregnation being realized inside the pore of the solid support. The increasing of the amplitude insures a higher stability of impregnation.
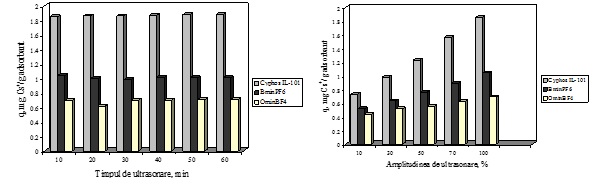
The optimum conditions of impregnation of a solid support with a ionic liquid through ultrasonication is the use of an amplitude of 100% for 10 minutes.
Objective 1.2. Characterization of the solids support impregnated with various ionic liquids
All the materials obtained through impregnation of various solid supports with various ionic liquids using various methods of impregnation were characterized by Fourier transform infrared spectroscopy FTIR, energy dispersive X-ray analysis (EDX), scanning electron microscopy (SEM) in order to confirm that the impregnation occurred, and in order to determine the morphological changes of the solid support surface.
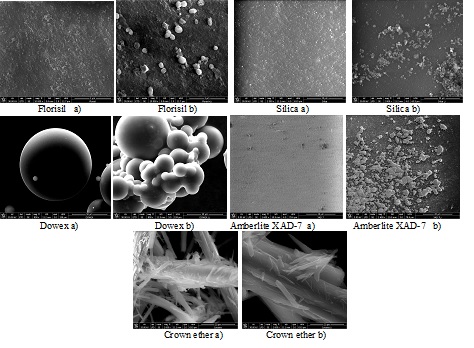
SEM images of different solid supports impregnated with CyphosIL-101 before(a) and (b) after impregnation
The characteristics of the obtained materials using various method of impregnation results from the EDX and BET analyze
|
Suportul anorganic / metoda de impregnare |
P, wt % |
Cl, wt % |
Suprafată specifică BET, m2/g |
Volumul porilor, cm3/g |
|
Florisil: - Ultrasonare - Metoda umedă - Impregnare prin agitare - Metoda uscată |
0.66 0.33 0.27 0.28 |
2.29 1.14 0.65 0.67 |
205.71 116.12 114.24 101.21 138.07 |
0.33 0.22 0.22 0.20 0.32 |
|
Silica: - Ultrasonare - Metoda umedă - Impregnare prin agitare - Metoda uscată |
0.54 0.28 0.23 0.24 |
0.96 0.72 0.44 0.45 |
262.10 202.77 201.25 168.64 210.50 |
0.74 0.53 0.56 0.42 0.58 |
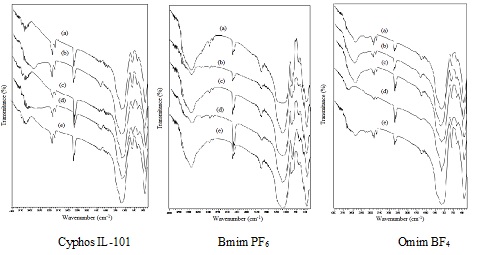
FTIR spectrums of Florisil impregnated with various ionic liquids at different amplitude of ultrasonication a) 10 %; b) 30 %; c) 50 %; d) 70 %; e) 100 %.
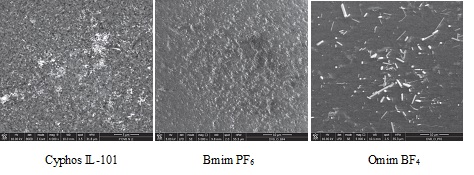
SEM image of functionalized polymer impregnated with various ionic liquids
Objective 2.1. Use of different ionic liquids for the removal of radionuclides from aqueous solutions
The purpose of this objective is to determine the influence of the ionic liquid nature, alchil chain length and nature of the used solvent upon the adsorption performance of the obtained materials. In this aim were made studies regarding the removal of Cs+, Sr2+ and Tl+ ions from aqueous solutions using Florisil impregnated with phosphonium based ionic liquids and imidazolium based ionic liquids. The same ionic liquids were used for the removal of Sr2+ and Tl+ ions from aqueous solutions but these ionic liquids were impregnated onto a functionalized polymer. It was observed than when an inorganic solid support was used the phosphonium based ionic liquid presented a higher efficiency in the removal process of radionuclides from aqueous solutions. When was used as solid support the functionalized polymer the imidazolium based ionic liquids showed better performance. These could be explained by the synergic effects, the solid support has the same functional element as the impregnated ionic liquid.
In order to determine the influence of the alkyl chain length were used three ionic liquid, having the same anion but different alkyl chain: 1 ethyl – 3 methyl imidazolium chloride (symbolized C2); 1 butyl – 3 methyl imidazolium chloride (symbolized C4) and 1 hexyl – 3 methyl imidazolium chloride (symbolized C6). Were used three solvents acetone, ethylic alcohol and toluene. All these ionic liquids were impregnated onto Florisil. The obtained material were also characterized and used in the removal process of various radionuclides (Cs+, Sr2+, Eu3+, La3+, Tl+ şi Nd3+) from aqueous solutions.
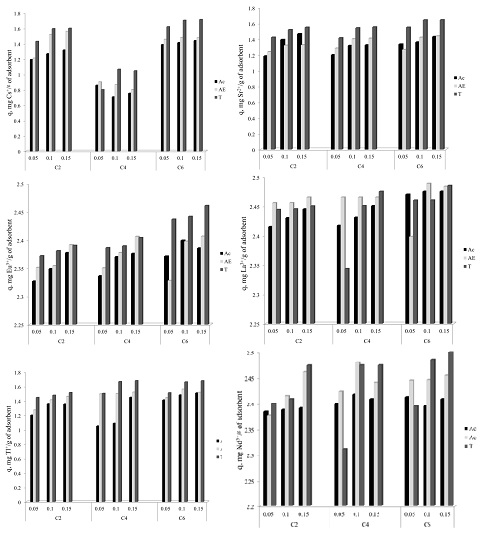
It can be observed that the adsorption capacities of the radionuclides from the aqueous solutions are strongly influenced by the nature of the ionic liquid, by the nature of the solvent and a less influence has the quantity of the ionic liquid impregnated onto the solid support. It can be observed that the adsorption capacity of the studied material increase with the increasing of the alkyl chain length from the used ionic liquid to the impregnation. Also the most efficient solvent proved to be toluene.
Objective 2.2. The removal of various radionuclides from aqueous solutions (Batch experiments)
In order to determine the efficiency of the obtained ionic liquid impregnated solid support, these were used in the removal process of radionuclides from aqueous solutions. The influence of various physical-chemical parameters (quantity of the ionic liquid impregnated, pH of solution, solid:liquid ratio, stirring time, temperature and initial concentration of the radionuclides from aqueous solutions) upon the adsorption performance of the obtained material in the removal process of radionuclides from aqueous solutions, was determined. It was observed that is not recommended to impregnate a higher quantity of ionic liquid onto 1 g of solid suprt than 0.1 g. At higher quantity of IL impregnated onto a solid support the obtained impregnated materials become sticky and form some conglomeration due to the viscosity of the IL which lead to the decreasing of the specific surface area and also to the decreasing of the maximum adsorption capacity. In order to obtain a higher adsorption capacity and a higher removal degree is not necessary to use a higher amount of ionic liquid impregnated solid support. The optimum solid:liquid ratio proved to be 0.1 g in 25 mL of aqueous solutions containing radionuclides.
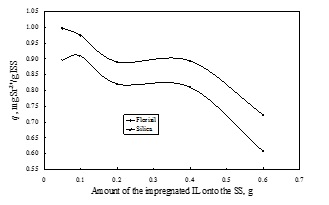
The influence of the quantity of ionic liquid impregnated onto the solid support (dry method) on the adsorption capacity of Sr2+ from aqueous solutions
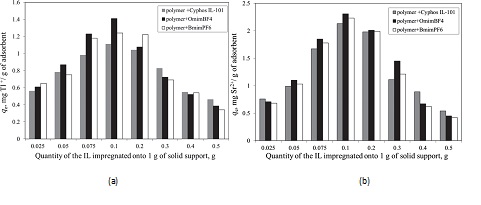
The influence of the quantity of ionic liquid impregnated onto the solid support (functionalized polymer using ultrasonication method) on the adsorption capacity of Tl+(a) and Sr2+(b) from aqueous solutions
The adsorbed amount of the radionuclides ions increase with the increase in their initial concentration. This is expected because at higher initial concentration more efficient utilization of active sites is envisaged due to a greater driving force by a higher concentration gradient. The collision between the metal ions and the active sites of the adsorbent is the major factor in kinetics which can lead to the increase in the rate of chemical reactions. This effect is positively influenced by the increasing of the initial concentration, which lead to a higher availability of metals ions for sorption. These sorption characteristics indicate that surface saturation is dependent on the initial metal ion concentration, as this determines the amount of metal ions adsorbed by the impregnated solid support in the presence of available active sites. The data deduced from the effect of metal ions initial concentrations were used to obtain various adsorption isotherms, which gives important data like: adsorption capacity of the studied materials and equilibrium coefficient for adsorption that could be used for adsorption design purpose. In the present study four adsorption isotherm models were utilized: Langmuir, Freundlich, Temkin and Dubinin-Radushkevich. These isotherms are useful for comparing results from different sources on a quantitative basis, providing information on the adsorption potential of a material with easily interpretable constants. K T was used to determine the value of the Gibbs free energy of adsorption.
Parametres of equilibrium isotherms for Cs+ adsorption onto Florisil impregnated with BmimPF6 using ultrasonication method of impregnation
|
Langmuir isotherm |
Freundlich isotherm |
|||||
|
qm exp, mg/g |
qm calc, mg/g |
KL, L/mg |
R2 |
KF, mg/g |
1/n |
R2 |
|
1.47 |
1.6 |
0.24 |
0.9990 |
1.96 |
0.30 |
0.9063 |
|
Temkin isotherm |
D-R isotherm |
|||||
|
KT, L/g |
bT |
∆Go |
R2 |
qd, mg/g |
E, kJ/mol |
R2 |
|
3.63 |
8.26 |
- 3.19 |
0.9624 |
1.36 |
14.2 |
0.9575 |
In order to determine the contact time between the adsorbent and adsorbate, kinetic studies were used. Adsorption kinetics was evaluated at three different temperatures. The equilibrium between adsorbent and adsorbate is achieved in a shorter time than in the case of other adsorbent reported in literature. At higher stirring times the adsorption capacity becomes linearly constant. This may be due the overlapping of active sites with metal iosn species and also due to decrease in the effective surface area resulting in the conglomeration of exchange particle. Adsorption capacity increase with the temperature, this indicates that the adsorption is endothermic in nature.
Pseudo-first-order equation and pseudo-second-order reaction model were used in order to study the radionuclides adsorption mechanism onto the ionic liquid impregnated materials.
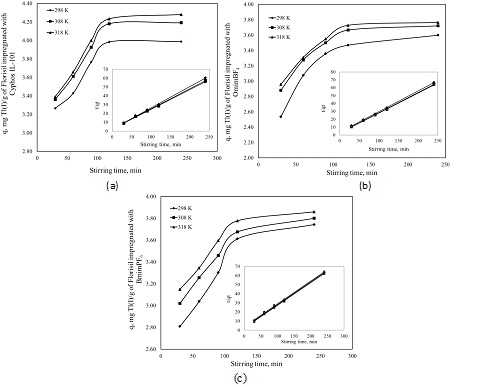
Effect of stirring time on the adsprtion capacity of Florisil impregnated with various ionic liquid in the removal process of Tl+ from aqueous solutions
(a) Florisil impregnated with Cyphos IL-101; (b) Florisil impregnated with OmimBF4; (c) Florisil impregnated with BmimPF6.
Thermodynamic parameters like activation energy, Gibbs free energy (ΔG), entropy (ΔS) and heat of adsorption (enthalpy ΔH) were also, evaluated.
Thermodynamic parameters for Tl+ adsorption onto the studied material
|
Adsorbent |
Activation energy |
Thermodynamic parameters |
|||||
|
ΔH°, kJ/mo-1 |
ΔS°, J/(mol·K) |
ΔGo, kJ/mol |
R2 |
||||
|
E, kJ/mol |
298 K |
303 K |
308 K |
||||
|
Florisil impregnated with Cyphos IL-101 |
8.5 |
15.4 |
57.6 |
-1.77 |
-2.39 |
-2.92 |
0.9791 |
|
Florisil impregnated with OmimBF4 |
13.5 |
8.62 |
31.1 |
-0.65 |
- 0.96 |
-1.27 |
0.9994 |
|
Florisil impregnated with BmimPF6 |
18.26 |
5.35 |
21.3 |
-0.99 |
-1.21 |
-1.42 |
0.9856 |
For all the studied adsorbents the positive values of ΔH0 indicate the entdothermic nature of metal ions adsorption. All ΔG0 values are negative and the Gibbs free energy decrease with the temperature increasing indicating the spontaneous nature of the metal ions sorption onto solid supportl impregnated with various ionic liquids. The positive value of ΔSo suggested an increase in randomness at the solid/liquid interface during adsorption of metal ions onto the studied materials.
In case of Sr2+ ions adsorption using Florisil impregnated with Cyphos IL-101 through ultrasound method of impregnation the adsorption process was optimised using the Factorial Design method.
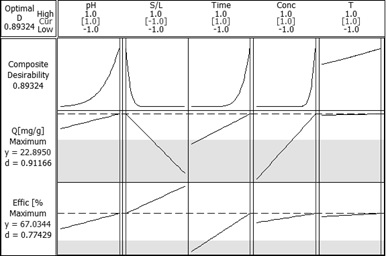
Response optimization for Sr2+ adsorption onto Florisil impregnated with Cyphos IL-101
Objective 3.1. The removal of various radionuclides from aqueous solutions using dynamic regim
The studies conducted in batch system allowed the establishment the equilibrium isotherm and the maximum adsorption capacity of the studied adsorbents in the removal process of radionuclides from aqueous solutions. In order to transfer the adsorption process of radionuclides, onto ionic liquids impregnated solid support, from laboratory scale to pilot scale it is necessary to conduct the experiments in fixed-bed column. Even if this method of separation implies large physical area needed for operation and higher capital cost investment present some advantages such as: little operator attention, easy inspection and cleaning for regeneration of adsorbent, and fewer instances of adsorbent particles in the effluent.
Activity 3.1.1. The influence of different physical - chemical parameters
The effect of flow rate on the breakthrough curve of the Sr2+ adsorption onto Florisil Impregnated with Cyphos IL-101 was studied by varying the flow rate from 3 to 7 mL/min, with constant bed depth (of 3 cm) and a concentration of Sr2+ of 20 mg/L.
Breakthrough curve for Sr2+ adsorption at different flow rate,
C0=20 mg/L; h=3 cm
Parameters of the fixed-bed column for Sr2+ adsorption onto ionic liquid impregnated Florisil at different flow rate
|
Flow rate, mL/min |
Bed volume, cm3 |
tb, min |
Exhausted time, min |
Vb, mL |
Treated volume, mL |
EBCT, min |
Mass of adsorbent, g |
Usage rate, g/mL |
|
3 |
9.42 |
133 |
217 |
400 |
650 |
3.14 |
8.3 |
0.0208 |
|
5 |
9.42 |
30 |
110 |
150 |
550 |
1.88 |
8.3 |
0.0553 |
|
7 |
9.42 |
11.4 |
57.1 |
80 |
400 |
1.35 |
8.3 |
0.104 |
The results indicate that an increase in flow rate at constant bed depth lead to the decrease of the breakhtrough time, of the exhaustion time and of the breakthrough volume (Vb) due to a decrease in Empty Bed Contact Time (EBCT). This hapened because with the flow rate increasing, the contact time between the adsorbent and adsorbate decrease. Therefore the adsorption capacity decrease due to the the decreasing of stationary time of the adsorbate between the adsorbent particles, and the adsorbent has not enough time to bound efficintly the metals molecules. The flow rate which lead to the most eficient adsorption of Sr2+ ions onto ionic liquid impregnated Florisil is 3 mL/min.
The breakthrough curves of Sr2+ adsorption onto ionic liquid impregnated Florisil using various bed depth at a constant flow rate of 3 mL/min was also studied. The results show that the slope of the breakthrough curve decrease with the bed depth incresing. With the bed depth increasing, increase the quantity of the used adsorbent, increase the active sites for the adsorption, increase the contact time between the adsorbate and adsorbent which lead to the increasing of the breakthrough and exahusted time, lead to the increasing of the breakthrough volume and the total treated volume. The most efficient bed depth for Sr2+ adsorption onto ionic liquid impregnated Forisil is 5 cm.
Breakthrough curve for Sr2+ adsorption at different bed depth,
C0=20 mg/L; Q=3 mL/min
Parameters of the fixed-bed column for Sr2+ adsorption onto ionic liquid impregnated florisil at different bed depth
|
Bed depth, cm |
Bed volume, cm3 |
tb, min |
Exhausted time, min |
Vb, mL |
Treated volume, mL |
EBCT, min |
Mass of adsorbent, g |
Usage rate, g/mL |
|
2 |
6.28 |
83.3 |
187 |
250 |
560 |
2.09 |
5.5 |
0.0220 |
|
3 |
9.42 |
133 |
217 |
400 |
650 |
3.14 |
8.3 |
0.0208 |
|
5 |
15.7 |
187 |
287 |
560 |
860 |
5.23 |
13.8 |
0.0246 |
The effect of the Sr2+ initial concentration on the breakthrough curve of Sr2+ adsorption was determined at a flow rate of 3 mL/min and a bed depth of 5 cm.
Breakthrough curve for Sr2+ adsorption at initial concentration of Sr2+,
Q=3 mL/min, h=5 cm
Parameters of the fixed-bed column for Sr2+ adsorption onto ionic liquid impregnated florisil at different Sr2+ initial concentrations
|
C0, mg Sr2+/L |
Bed volume, cm3 |
tb, min |
Exhausted time, min |
Vb, mL |
Treated volume, mL |
EBCT, min |
Mass of adsorbent, g |
Usage rate, g/mL |
|
20 |
15.7 |
187 |
287 |
560 |
860 |
5.23 |
13.8 |
0.0246 |
|
35 |
15.7 |
110 |
217 |
330 |
650 |
5.23 |
13.8 |
0.0418 |
|
50 |
15.7 |
33.3 |
170 |
100 |
510 |
5.23 |
13.8 |
0.138 |
Activity 3.1.2. Column modeling
For the estimtion of the maximum adsorption capacity and kinetic constant of an adsorption process in dinamic regim untill the breakthroguh occur the experimental data was modeled using BDST model which predict the relationship between service time and bed depth and the linear relationship is given by Eq. 1:
where: C0 represent the initial concentration of Sr2+ in the solution, mg/L, CB the Sr2+ residual concentration at breakthrough, mg/L, K the adsorption rate constant, L/mg/h, N0 the adsorption capacity of Sr2+ onto the Florisil impregnated with Cyphos IL-101, X the bed depth of adsorbent in the column, cm, ν is the flow velocity, cm/h, t is the service time of column under above conditions, hr.
From the slope and intercept of the linear plot of t=f(X) can be calculated the adsorption capacity (N0) and the adsorption rate constant K for the Sr2+ adsorption onto ionic liquid impregnated Florisil.
.jpg)
BDST model of Sr2+ adsorption onto ionic liquid impregnated Florisil
The value of the correlation coeficient indicates the validity of the BDST model for present column system. The values of K and N0 indicated that the Sr2+ can be efficiently removed through adsorption onto ionic liquid impregnated Florisil. The maximum amount of Sr2+ which can be adsorbed by 1 g of ionic liquid impregnated Florisil can be calculated by dividing N0 with the packed density of ionic liquid impregnated Florisil in column (0.88 g/cm3).
The constants of BDST model for Sr2+ adsorption onto ionic liquid impregnated Florisil
|
Flow rate, mL/min |
Linear flow rate, cm/hr |
N0, mg/cm3 |
K, cm3/ mg hr |
R2 |
qm, mg/g |
|
3 |
57.32 |
38.38 |
10.94 |
0.972 |
43.6 |
Objective 3.2. Effect of the competitives cations
In order to determine the selectivity of the studied adsorbent, the influence of the presence of Ca2+ and K+ ions at different concentration was studied and the breakthrough curves were ploted. It can be observed that, when the Ca2+ ions are present in solution at a concentration lower or equal with the concentration of Sr2+ ions the adsorption efficiency of the Florisil impregnated with Cyphos IL-101 is not significantly influenced, but when the concentration of Ca2+ is higher than the concentration of Sr2+ the adsorbtion efficiency of the column decrease. It can be notice that the Florisil impregnated with Cyphos IL-101 has a higher selectivity for Sr2+ ions compared with Ca2+. For all the studied case, the breakthrough time and the exahusted time for Ca2+ adsorption are lower than those for Sr2+ adsorption. When Sr2+ ions are present in solution together with the K2+ ions the behaviour of the column, feed with impregnated Florisil, in the removal process of Sr2+ ions from aqueous solutions, is similar like in the absence of the K+ ions. From this study also can be conclude that the selectivity of the studied adsorbent is higher for Sr2+ adsorption than for K+ adsorption.
.jpg)
Breakthrough curve for Sr2+ and Ca2+ adsorption onto Florisil impregnated with Cyphos IL-101, when both ions are present in solution
.jpg)
Breakthrough curve for Sr2+ and K+ adsorption onto Florisil impregnated with Cyphos IL-101, when both ions are present in solution
The breakthrough curve for all 3 studied cations, present toghether in solution at the same concentration of 20 mg/L were also plotted. It can be observed that the breakthrough volume from 560 mL to 520 mL, and the treated volume 860 mL to 810 mL. It can be mentioned that even if in solution are all three cations, the behaviour of Sr2+ adsorption is not significantly influenced, the behaviour is similar like in the case of the presence just of Ca2+ ions. Toghether with the Sr2+ ions are adsorbed the K+ ions and in a smaller ratio the Ca2+. It can be conclude that the studied adsorbent has a high affinity for the Sr2+ adsorption from aqueous solutions.
Breakthrough curve for Sr2+, Ca2+ and K+ adsorption onto Florisil impregnated with Cyphos IL-101, when all ions are present in solution
Parameters of the fixed-bed column for Sr2+ adsorption onto ionic liquid impregnated florisil in the present of competitive ions
|
C0, mg Sr2+/L |
C0, mg Ca2+/L |
C0, mg K+/L |
tb, min |
Exhausted time, min |
Vb, mL |
Treated volume, mL |
Usage rate, g/mL |
|
20 |
0 |
0 |
187 |
287 |
560 |
860 |
0.0246 |
|
20 |
10 |
0 |
187 |
270 |
560 |
810 |
0.0246 |
|
20 |
20 |
0 |
177 |
270 |
530 |
810 |
0.0260 |
|
20 |
30 |
0 |
160 |
233 |
480 |
700 |
0.0288 |
|
20 |
0 |
10 |
187 |
287 |
560 |
860 |
0.0246 |
|
20 |
0 |
20 |
187 |
287 |
560 |
860 |
0.0246 |
|
20 |
0 |
30 |
187 |
270 |
560 |
810 |
0.0246 |
|
20 |
20 |
20 |
173 |
270 |
520 |
810 |
0.0265 |
Objective 4.1. Radionuclides desorption form the solid supports impregnated with ionic liquids.
The column used for the adsorption process of Sr2+ in the optimum conditions: C0 = 20 mg Sr2+/L, Q=3 mL/min, h=5 cm, was regenerated with 5% HCl solution. The desorption process was carried out with the same flow velocity of 3 mL/min. From the desorption curve of Sr2+ adsorbed on Florisil impregnated with Cyphos IL-101 it can be observed that with 100 mL of 5% HCl solution is realized a desorption over 93% of the adsorbed Sr2+ onto Florisil impregnated with Cyphos IL-101. The column is regenerated in a shorter time than is exhausted, and is required a small volume of the eluent for Sr2+ desorption obtaining a concentrated solution of Sr2+, which is important from the reuse and capitalization point of view. The column was used for 4 cycles of adsorption/desorption process of Sr2+ from aqueous solutions using as adsorbent the Florisil impregnated with Cyphos IL-101 (activity 4.1.2). It can be notice that, after the 1st and 2nd regeneration process the adsorption behaviour of the column, feed with Florisil impregnated with Cyphos IL-101, is quite similar with the fresh column in the removal process of Sr2+ ions from aqueous solutions. In the 1st and 2nd regeneration process the desorption of Sr2+ from the saturated column, using 5% HCl solution, is done with a removal rate over 90%. After the 3rd desorption process, the removal rate of Sr2+ decrease, therefore the adsorption efficiency of the regenarate adsorbent decrease also with 20%.
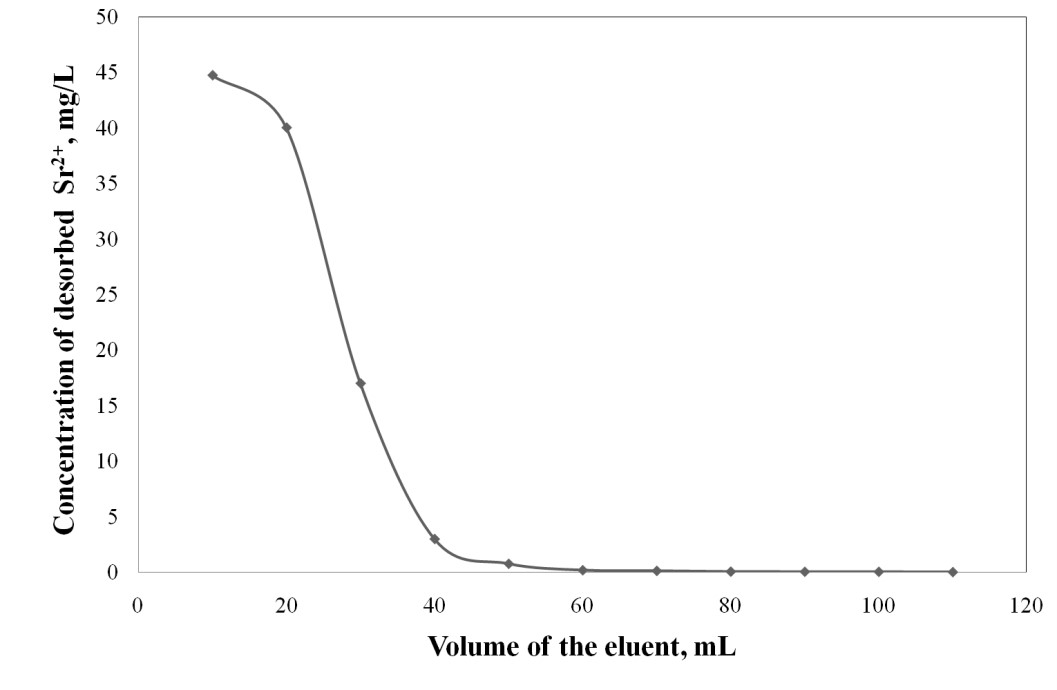
Desorption curve of Sr2+ from the exhausted adsorbent
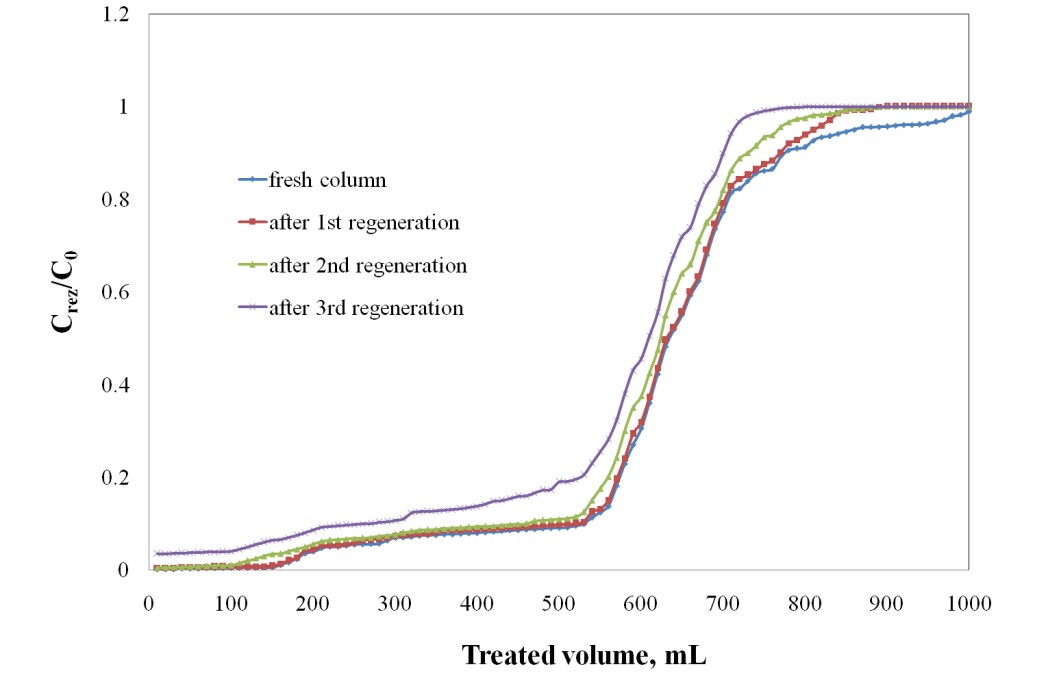
Reuse of the regenerated column in the removal process of Sr2+ ions from aqueous solutions
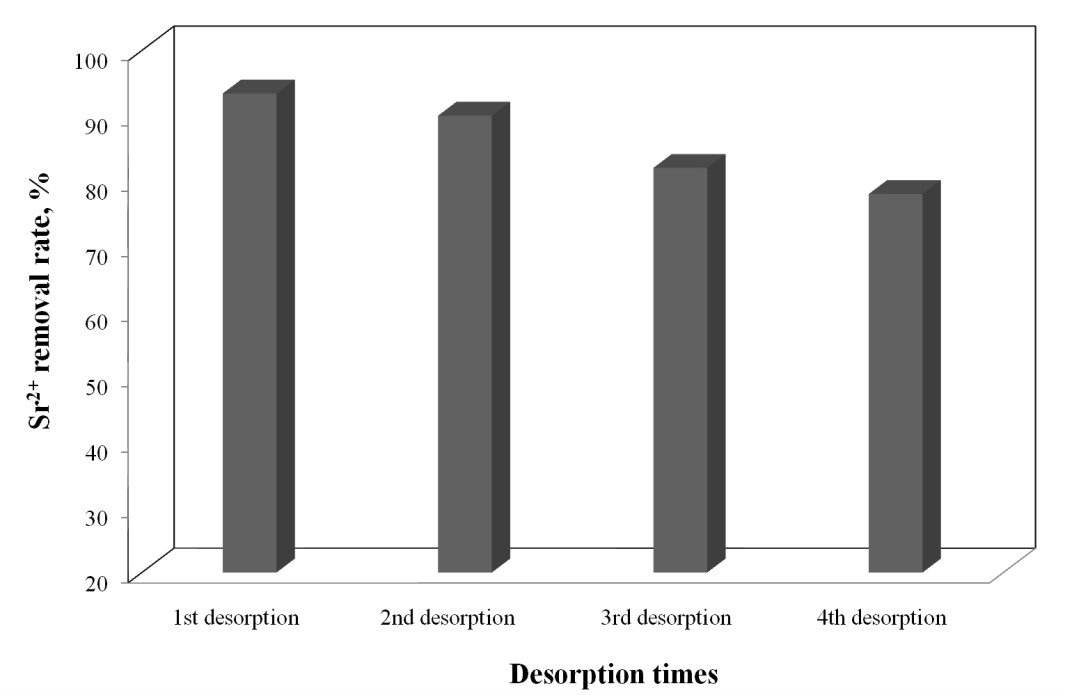
The desorption degree of Sr2+ ions in the regeneration processes
RESULTS-PUBLICATIONS
2013
Published articles in ISI indexed journals
- L. Lupa, A. Negrea, M. Ciopec, P. Negrea, Cs+ removal from aqueous solutions through adsorption onto Florisil impregnated with trihexyl(tetradecyl) phosphonioum chloride, Molecules, 2013, 18(10), 12845-12856; doi:10.3390/molecules181012845; http://www.mdpi.com/1420-3049/18/10/12845;
- A. Negrea, L. Lupa, M. Ciopec, P. Negrea, R. Vodă, C. Ianasi, Study of different impregnation methods of inorganic support with ionic liquid, Journal of Environmental Protection and Ecology, 2013, 14(4), 1785-1793;
Published articles in BDI indexed journals
- A. Negrea, M. Ciopec, L. Lupa, P. Negrea, A. Gabor, Influence of the solid support base impregnated with IL on the sorption of various radionuclides from aqueous solutions, AWERProcediaAdvences in Applied Science, 2013, 1, 241-250;
- A. Negrea, L. Lupa, M. Ciopec, P. Negrea, Characterization of strontium adsorption from aqueous solutions using inorganic materials impregnated with ionic liquid, International Journal of Chemical Engineering and Applications, 2013, 4(5), 326-331;
Presented articles at international conferences
- A. Negrea, L. Lupa, M. Ciopec, P. Negrea, R. Vodă, C. Ianasi, Study of different impregnation methods of inorganic support with ionic liquid, Intenrational U.A.B. – B.E.N.A. Conference, Environmental Engineering and Sustainable Development, 23-25 Mai 2013, Alba Iulia, Romania;
- A. Negrea, L. Lupa, M. Ciopec, P. Negrea, Silica impregnated with Cyphos IL-101 for Cs+ adsorption, 7th International Conference – Environmental Engineering and Management, Integration challenges for Sustainability, 18-21 Septembrie, Viena, Austria;
- M. Ciopec, A. Negrea, P. Negrea, L. Lupa, Use of SiO2 impregnated with crown ether and ionic liquid as new adsorbent materials in the removal process of Cs+ from aqueous solutions, The 19th International Symposium on Analytical and Environmental Problems, 23 Septembrie 2013, Szeged, Ungaria;
- A. Negrea, L. Lupa, M. Ciopec, P. Negrea, Characterization of strontium adsorption from aqueous solutions using inorganic materials impregnated with ionic liquid, 4th International Conference on Chemical Engineering and Applications (CCEA 2013), 12-13 Octombrie 2013, Paris, Franta;
- M. Ciopec, A. Popa, A. Negrea, L. Lupa, P. Negrea, R. Voda, C.M. Davidescu, G. Ilia, Comparative characteristics of some material polymers impregnated with ionic liquid for removal of radionuclides, International Conference – Environmental Research and Technology, ECOIMPULS 2013, 7-8 Noembrie 2013, Timisoara, Romania.
2014
Published articles in ISI indexed journals
- A. Popa, M. Ciopec, A. Negrea, L. Lupa, P. Negrea, C.M. Davidescu, G. Ilia, N. Duteanu, Use of styrene-divinylbenzene grafted with aminoethylaminomethyl groups and various ionic liquids in the removal process of thallium and strontium, Pure and Applied Chemistry, 2014, 86(11), 1741-1753, DOI 10.1515/pac-2014-0702, http://www.degruyter.com/view/j/pac.2014.86.issue-11/pac-2014-0702/pac-2014-0702.xml;
- A. Negrea, L. Lupa, M. Ciopec, P. Negrea, Silica impregnated with Cyphos IL-101 for Cs+adsorbtion, Environmental Engineering and Management Journal, 2014, 13(8), 2005-2013;
Published articles in BDI indexed journals
- A. Negrea, L. Lupa, M. Ciopec, R. Voda, P. Negrea, I. Hulka, Ultrasonication impregnation of Florisil with 1-octyl-3-methylimidazolium tetrafluoroborate used as an adsorbent in the removal process of Cs+ from aqueous solutions, WIT Transactions on Ecology and The Environment, 2014, 182, 223-232; DOI: 10.2495/WPI40201.
- A. Negrea, L. Lupa, M. Ciopec, P. Negrea, I. Hulka, Studies regarding the Florisil impregnation with ionic liquid through ultrasonication, International Journal of Chemical Engineering and Aplications, 2014, 5(5), 424-428; DOI:10.7763/JCEA.2014.V5.422
Presented articles at international conferences
- A. Negrea, L. Lupa, M. Ciopec, P. Negrea, I. Hulka, Studies regarding the Florisil impregnation with ionic liquid through ultrasonication, „2014 International Conference on Chemical and Food Engineering”, Dubai, EmirateleArabe Unite, 04.04.2014 – 05.04.2014;
- A. Negrea, L. Lupa, M. Ciopec, R. Voda, P. Negrea, I. Hulka, Ultrasonication impregnation of Florisil with 1-octyl-3-methylimidazolium tetrafluoroborate used as an adsorbent in the removal process of Cs+ from aqueous solutions, „Water Pollution 2014”, Algarve, Portugalia, 25.05.2014 – 29.05.2014;
- A. Popa, M. Ciopec, A. Negrea, L. Lupa, P. Negrea, C.M. Davidescu, G. Ilia, N. Duteanu, Use of styrene-divinylbenzene grafted with aminoethylaminomethyl groups and various ionic liquids in the removal process of thallium and strontium, „Polymer and Organic Chemistry 2014”, Timisoara, Romania, 10.06.2014 – 13.06.2014;
- A. Negrea, L. Lupa, M. Ciopec, P. Negrea, R. Voda, Ionic liquids impregnated onto inorganic support used for thallium adsorption from aqueous solutions, „2nd International Conference on Ionic Liquids in Separation and Purification Technology”, Toronto, Canada, 29.06.2014 – 02.07.2014;
- L. Lupa, A. Negrea, M. Ciopec, R. Voda, P. Negrea, Effects of ultrasonication conditions of ionic liquid impregnated solid support preparation on subsequent adsorption of Cs+ ions from aqueous solutions, „Environmental Legislation, Safety Engineering and Disaster Management”, Cluj-Napoca, 18.09.2014-20.09.2014.
2015
Published articles in ISI indexed journals
- M. Ciopec, A. Popa, A. Negrea, L. Lupa, P. Negrea, R. Voda, C.M. Davidescu, G. Ilia, Comparative characteristics of some material polymers impregnated with ionic liquid for removal of radionuclides, Environmental Engineering and Management Journal, 2015, 14(6), 1287-1294;
- L. Lupa, A. Negrea, M. Ciopec, P. Negrea, R. Voda, Ionic liquids impregnated onto inorganic support used for thallium adsorption from aqueous solutions, Separation and Purification Technology, 2015, 155, 75-82, doi.org/10.1016/j.seppur.2015.06.043.
Published articles in BDI indexed journals
- Lupa, A. Negrea, M. Ciopec, R. Voda, P. Negrea, Studies regarding the influence of the ultrasonication conditions on the adsorption performance of obtained ionic liquid impregnated materials, International Journal of Chemical Engineering and Applications, 2015, 6(6), 410-416, DOI: 10.7763/IJCEA.2015.V6.520
Presented articles at international conferences
- Lupa, A. Negrea, M. Ciopec, R. Voda, P. Negrea, Studies regarding the influence of the ultrasonication conditions on the adsorption performance of obtained ionic liquid impregnated materials, 2nd International Conference on Chemical and Biological Sciences (ICCBS 2015), Florenta, Italia, 19.03. – 20.03, 2015;
- L. Lupa, P. Negrea, Radionuclides removal using ionic liquids impregnated materials, The 8th edition of symposium with international participation “New trends and strategies in the chemistry of advanced materials with relevance in biological systems, technique and environmental protection”, Timisoara, Romania, 04.06.-05.06., 2015;
- L. Lupa, A. Popa, R. Voda, P. Negrea, M. Ciopec, A. Negrea, Strontium adsorption on ionic liquid impregnated Florisil. Fixed-Bed column studies, IIIrd International Conference on Methods and Materials for Separation Processes, Separation Science – Theory and Practice, Karpacz, Polonia, 06.09. – 10.09. 2015;
- L. Lupa, A. Popa, E.S. Dragan, M. Ciopec, A. Negrea, P. Negrea, Performance improvement of adsorption using the organic solid support impregnated with ionic liquid in the removal process of Tl(I) from aqueous solutions, 8th International Conference on Environmental Engineering and Management, Iasi, Romania, 09.09. – 12.09. 2015;
- P. Negrea, L. Lupa, A. Popa, M. Stoia, Studies Regarding Strontium Adsorption onto Styrene-1% Divinylbenzene Grafted with Phosphonium Groups and Impregnated with Ionic Liquid, 21st International Symposium on Analytical And Environmental Problems, Szeged, Ungaria, 28.09.2015.
2016
Published articles in ISI indexed journals
- L. Lupa, A.Popa, R. Voda, P. Negrea, M. Ciopec, A. Vasile, Strontium adsorption on ionic liquid impregnated Florisil: Fixed-bed column studies, Separation Science and Technology, published on-line: April 2016; DOI: 10.1080/01496395.2016.1171240;
- P. Negrea, A. Popa, L. Lupa, R. Voda, Thallium removal through adsorption onto ionic liquid impregnated solid support. Influence of the impregnation conditions, International Journal of Environmental Science and Technology, 2016, 13, 1873-1882; DOI: 10.1007/s13762-016-1017-0;
- L. Lupa, A. Popa, E.S. Dragan, M. Ciopec, A. Negrea, P. Negrea, Adsorption performance of the organic solid support impregnated with ionic liquid in the removal process of Tl(I) from aqueous solutions, Process Safety and Environmental Protection, published on-line: August 2016, DOI: 10.1016/j.psep.2016.08.015.
- M. Ciopec, A. Negrea I. Grozav, L. Lupa, P. Negrea, Optimization of strontium adsorption using Florisil impregnated with ionic liquid by factorial design, International Journal of Environmental Science and Technology, under review;
- M. Ciopec, L. Lupa, P. Negrea, A. Negrea, The influence of the ionic liquid and solvent nature used for the impregnation of Florisil on the performance of the solid-phase extraction of radionuclides, Separation and Purification Technology, under review;
- L. Lupa, R. Voda, A. Popa, Adsorption behavior of cesium and strontium onto chitosan impregnated with ionic liquid, Separation Science and Technology, under review.
Presented articles at international conferences
- L. Lupa, R. Voda, A. Popa, Adsorption behavior of cesium and strontium onto chitosan impregnated with ionic liquid, 4th International Conference on Methods and Materials for Separation Processes, Separation Science – Theory and Practice, Brunow, Polonia, 04.09. – 08.09. 2016.
Patent Demand
- L. Lupa, P. Negrea, A. Negrea, M. Ciopec, R. Voda, Method for radionuclides removal from aqueous solution using ionic liquid impregnated solid supports, No. A/00754. 23.10.2015, published in “Buletinul Oficial de Proprietate Industriala – sectiunea Brevete de Inventie”, nr. 6, 2016.
Published book chapter
- Lupa, P. Negrea, A. Popa, Use of ionic liquids in solid-liquid separation processes – 20 pages, Ionic Liquids, Book edited by: Dr. Scott Handy, Publishing House: InTech, ISBN: 978-953-51-4897-5.
Contact:
S.l. dr. eng. Lavinia LUPA
Politehnica University Timişoara
Tel./Fax: +40-256-404192/ +40-256-403060
Această adresă de email este protejată contra spambots. Trebuie să activați JavaScript pentru a o vedea.
Last update: 02.09.2016


