Project financed by UEFISCDI, Executive Agency for Higher Education, Research, Development and Innovation, IDEAS Programme, Exploratory Research Projects (PCE)
Host institution: Politehnica University Timişoara
Project registration code: PN-II-ID-PCE-2012-4-0398
Contract number: 77 / 02.09.2013
Project title: New fabrication concept of silver nanowire/polyaniline transparent, conductive and flexible electrodes for solar cells
Project summary: A great challenge in the actual research of solar-to-electricity conversion is the construction of flexible solar cells. Although indium tin oxide (ITO) deposited on plastic is traditionally used for organic solar cells and light emitting diodes, solutions are searched to replace the ITO layer and to manufacture cheap transparent conducting electrodes. Silver nanowires (AgNWs) are a promising candidate to replace ITO due to their high electric conductivity and corrosion resistance, but there is still the issue of increased resistance on wire contacts. The aim of the project is to develop transparent, conductive and flexible electrodes for solar cells based on silver nanowire/polyaniline hybrid materials and to offer a new technical solution to decrease the sheet resistance of the silver nanowires embedded in the polymer matrix.
Work plan
Main objectives:
1. Synthesis and characterization of silver nanowires with controlled aspect ratio (2013).
2. Development and characterization of transparent conductive electrodes on flexible substrates using silver nanowires and assessment of their electrical and optical properties (2014)
3. Synthesis and characterization of indium and tin nanoparticles (2014)
4. Synthesis and characterization of silver nanowires modified with tin and indium nanoparticles (2015)
5. Preparation of electroconductive inks based on silver nanowires (2015)
6. Optimization of silver nanowires-based flexible, transparent and conducting electrodes to increase diffuse transmittance / resistance ratio (2016)
7. Deposition of a conducting polymer on previously manufactured electrodes (2016)
8. Construction of dye-sensitized solar cells using silver nanowires-based transparent and conducting electrodes (2016).
Project start date: 02.09.2013
Project completion date: 05.12.2016
Total funding: 994,400 lei
2013: 91,300 lei
2014: 263,970 lei
2015: 300,761 lei
2016: 338,369 lei
Project manager: Assoc. Prof. Andrea Kellenberger
Team members:
Nyari Terezia senior researcher
Banica Radu Nicolae postdoctoral researcher
Dan Mircea Laurentiu PhD student
Locovei Cosmin postdoctoral researcher
Baies Radu postdoctoral researcher
Bucur Alin PhD student
Ursu Daniel Horatiu PhD student
Cseh Liliana senior researcher
Capota Paul Cristian master student
Vaszilcsin Nicolae senior researcher
Project status report 2013-2016
Objective 1 (2013): Solvothermal synthesis and morphological, structural and compositional characterization of silver nanowires with controlled aspect ratio.
The aim of this objective was the synthesis of silver nanowires (AgNWs) by the reduction of silver ions in ethylene glycol in the presence of surfactants. If the surfactant used is polyvinylpyrrolidone (PVP), the PVP : Ag molar ratio and Cl- ion concentration in the system have been shown to be essential for obtaining a reaction product rich in nanowires. To control the aspect ratio (diameter and length) of silver nanowires, the effect of synthesis conditions, such as injection temperature of Ag+ ions, rate of temperature increase of the reaction medium, molar ratio Ag : surfactant, temperature and synthesis time have been investigated.
The obtained silver nanostructures were characterized by X-ray diffraction, UV-Vis spectroscopy, scanning electron microscopy, energy dispersive X-ray analysis and transmission electron microscopy.
XRD patterns of the silver nanowires show the presence of cubic-phase Ag grown preferentially along the direction (111) as the major phase, together with tetragonal phase Ag. EDX analysis revealed the presence of silver, small amounts of chlorine due to traces of AgCl (consistent with XRD spectra) and carbon from the PVP adsorbed on the surface of nanowires. TG / DTG-ATD studies have shown the oxidative desorption of PVP in the temperature range 320-480°C. UV-Vis spectra show the an absorption maximum located at 378 nm atributed to plasmonic absorption of silver nanowires. An additional peak at 352 nm corresponds to macrocrytalline silver. Absorption maxima located at wavelengths of 385 nm is assigned to overlapped plasmonic absorptions of particles with different shapes, other than nanofibers. SEM micrographs show that regardless of the injection temperature of the precursor, silver nanowires with a very narrow size dispersion are obtained, the average diameter is about 50 nm in both cases. Injecting the precursor at 25°C leads to an average length of 3.5 to 4 μm, resulting in an average form factor (aspect ratio = L / D) of 80. Besides nanowires, nanoparticles with various shapes, essentially spherical and cubic are obtained, that can be easily removed by centrifugation. The average length of the nanowires obtained by the injection of precursor at 160°C is about 5 μm, resulting in a higher average form factor of 100. Also, the amount of silver nanoparticles having another shapes is lower, as a result we can conclude that the optimum temperature of the precursor injection is 160°C.
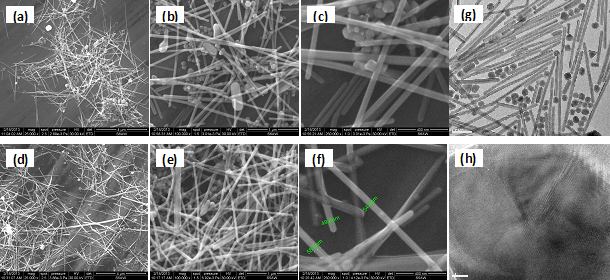
SEM micrographs of the synthesized silver nanowires obtained by injecting the precursor at 25°C (a - c) and 160°C (d - f). TEM images of silver nanowires and naoparticles with different shapes (g) and the bipyramidal pentagonal shape nucleation center of a nanowire (h).
Objective 2 (2014): Development and characterization of transparent conductive electrodes on flexible substrates using silver nanowires and assessment of their electrical and optical properties
Transparent and conductive electrodes have been obtained by depositing thin films of AgNWs on transparent polyethylene terephthalate (PET) sheets by doctor blade method. An intermediate thin layer of polymethylmethacrylate (PMMA) was applied to increase the adhesion of silver nanowires on PET support. In the next step, successive layers of AgNWs were deposited from a suspension of AgNWs in ethanol, also by means of doctor blade method with intermediate drying in an oven after each layer for 15 minutes at 50°C and cooling at room temperature another 15 to 20 minutes. By this procedure, samples with 2, 4 and respectively 6 layers of AgNWs have been prepared.

Images of transparent and conductive films based on silver nanowires obtained by depositing 2 layers (a), 4 layers (b) and 6 layers (c) of AgNWs on PET/PMMA.
The obtained silver nanostructures were characterized by X-ray diffraction (X'Pert PRO MPD PANalytical diffractometer, CuKα radiation, λ=1.54184 Å, Bragg-Bretano geometry), UV-Vis spectroscopy (Lambda 950 Perkin-Elmer spectrometer with integrating sphere) and scanning electron microscopy (FEI Inspect S). Sheet resistance of the transparent electrodes obtained from AgNWs deposited on PMMA/PET was determined by four point probe measurement using a Jandel RM3000 Test unit.

SEM micrographs of AgNWs films on PMMA/PET support for 2 (a), 4 (b) and 6 (c) succesive layers of AgNWs. UV-Vis diffuse transmittance spectra of PMMA/PET (a) and of AgNWs transparent films with 2 (b), 4 (c) and 6 (d) successive layers.
SEM images reveal that the deposition of two layers of AgNW on the substrate leads to the formation of isolated “islands” and “peninsulas” of silver nanowires unconnected or joined only through few nanowires. Increasing the number of deposited layers, these "islands" connect together electrically, leading to the formation of electrically conductive layers. The samples obtained by the deposition of 6 successive layers reveal an agglomerated, three-dimensional network structure of nanowires, composed of a large quantity of uniformly distributed nanowires. Diffuse transmittance spectra indicates good transparency in the visible, which decreases with increasing number of layers, reaching 75% for 2 layers, 65% for four layers and 58% for six layers of deposited AgNWs.
| Sample | 2 layers | 4 layers | 6 layers |
| Sheet resistance, Ω/sq | 554 | 41.25 | 31,47 |
Electrical measurements indicate good conductivities for samples with 4 and 6 layers of AgNWs. Sheet resistance can be further increased by 23 % by heating the film at 150°C for 10 minutes and 30 % by heating the film at 150°C for 40 minutes, due to improved electric contacts between the nanowires.
Objective 3 (2014): Synthesis and morphological, structural and compositional characterization of indium and tin nanoparticles
The synthesis of indium nanoparticles involves reduction of dissolved In (III) ions to In (0). The reduction is followed by nucleation, leading to the formation of metal nanoparticles. Since it is envisaged to obtain a narrow particle size distribution, the nucleation stept must be separed from the growth step. A strong and rapid supersaturation of the solution in indium leads to a high density of nucleation sites and reduced particle size. Increasing the nucleation sites density can be done mainly by two ways: (i) use an excess of one of the reactants; (ii) increasing the reaction rate by increasing temperature. Sodium borohydride was used as reducing agent and the reaction was conducted at high temperatures (100°C). Since In (0) nanoparticles are prone to oxidation, the synthesis was carried out under inert atmosphere (argon). To prevent agglomeration of nanoparticles a surfactant (trisodium citrate) was used. In(0) nanoparticles covered with trisodium citrate were synthesized according to the procedure described in the literature, leading to nanoparticles with a diameter in the range 10-20 nm. The dimension of nanoparticles was established by TEM and the size distribution by dynamic light scattering with a Nanosizer ZS; the morphology has studied by SEM. Moreover, the optical properties of nanoparticles were quantified by plasmon resonance absorption via UV-Vis. 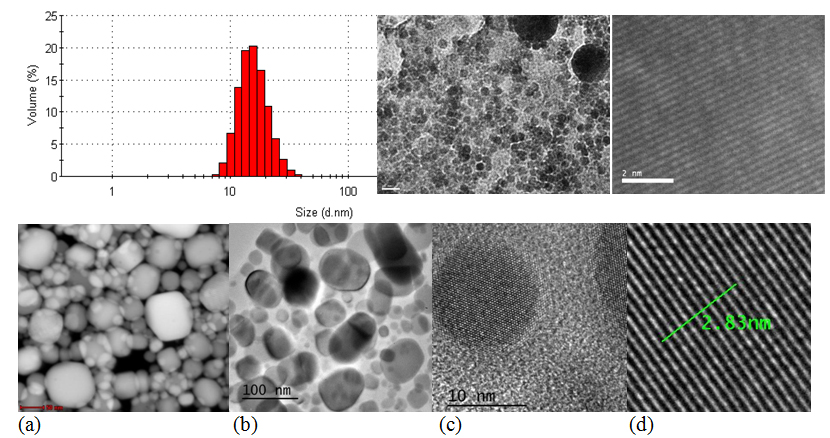
Fig. 21. Imagine STEM (a) , TEM (b), HR-TEM (c, d) a nanoparticulelor de staniu. In figura d, distanta de 2.83 nm corespunde distantei cumulate dintre 10 plane atomice. Asadar distanta interplanara este de cca 2.8 Ångström.
Objective 4 (2015): Synthesis and characterization of silver nanowires modified with tin and indium nanoparticles
In order to deposit In and Sn nanoparticles on silver nanowires, the method chosen was the precipitation of In and Sn nanoparticles in the presence of silver nanowires which may act as nucleation centers in a heterogeneous nucleation process. The corresponding metallic ions are dissolved in an aprotic solvent in the presence of a surfactant such as sodium citrate that prevents the agglomeration of particles. Then the suspension of silver nanowires is injected and finally the reducing agent (sodium borohydride) dissolved in a water-aprotic solvent mixture.
XRD profiles corresponding to modified silver nanowires shows that the only crystalline phase present is cubic metallic silver. Therefore, X-ray diffraction is not suitable to identify the decoration of silver nanowires with In and Sn nanoparticles, primarily due to the extremely low In(Sn)/Ag mass ratio.
TEM images indicate that the major part of indium that is decorating the silver nanowires consists of quasi-spherical particles having dimensions of 30-40 nm if the synthesis is conducted at high temperature and between 5-15 nm if the synthesis is performed at lower temperature. In case of Sn, the nanaoparticles have dimensions between 5-15 nm at low temperature synthesis. HR-TEM images indicate that In metal nanoparticles are covered with a layer of amorphous citrate. Between the monocrystalline core and the organic layer, the crystal lattice is dominated by structural defects, which implies a superficial oxidation of In nanoparticles. EDX maps unequivocally demonstrate that In and Sn nanoparticles are present on the surface of silver nanowires, and that during the reduction of In3+ ions it has not occurred any alloying process implying diffusion of In atoms in the silver nanowires. But this process takes place by the heat treatment of nanowires decorated with metal nanoparticles. Alloying of nanoparticles with silver nanowires has been observed mostly in case of large In nanoparticles, with diameters over 50 nm. For In/Sn nanoparticles with diameters between 15-20 nm the alloying does not take place due to the organic layers adsorbed on both nanoparticles and nanowires. The PVP layer on the surface of silver nanowires and the citrate layer on the surface of In/Sn nanoparticles prevents the alloying. Removing organic layers of PVP and citrate is not possible because it leads to desorption of In/Sn nanoparticles. The obtained results suggest that the strategy of welding nanowires with nanoparticles cannot be achieved by using decorated silver nanowires.
Objective 5 (2015): Preparation of electroconductive inks based on silver nanowires
For the preparation of inks and determination of the most appropriate dispersing media, the ethanol suspension of nanowires was subjected to vacuum evaporation at ambient temperature. The resulted solid mass was redispersed in different solvents (water, absolute ethanol, dichloroethane and n-hexane) obtaining different inks. The dielectric constants at 25°C of these solvents are 80.1, 24.5, 8.93 and 1.88, respectively. Polyethylene terephthalate (PET) and aluminum metal foil were used as substrates for the evaluation of the most suitable dispersion media. Another substrate (PETP) was manufactured by treating PET in oxygen plasma for 10 minutes. As working gas a mixture of 75% (vol.) Ar and 25% (vol.) O2 was used. The PET and Al substrates were washed before use with 98% ethanol. The PETP substrate was washed with ethanol only before the treatment in oxygen plasma.

SEM images of AgNW on Al support deposited from different solvents: water (a), ehtanol (b), dichloromethane (c) and n-hexane (d).
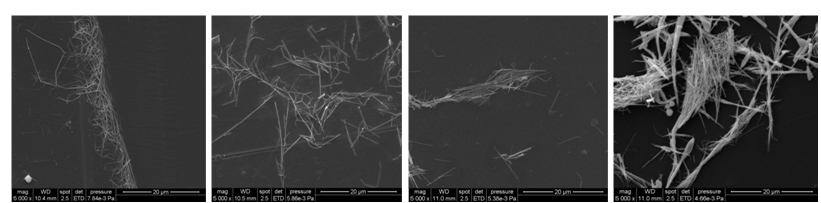
SEM images of AgNW on PET support deposited from different solvents: water (a), ehtanol (b), dichloromethane (c) and n-hexane (d).

SEM images of AgNW on PETP support deposited from different solvents: water (a), ehtanol (b), dichloromethane (c) and n-hexane (d).
As it was observed from SEM images, the use of water as solvent leads to the agglomeration of nanowires at the water-air interface in case of aluminum and untreated PET supports. But, the silver nanowires deposited on PETP support by using water as a dispersion medium are more uniformly distributed due to the much higher wettability of the plasma-treated polymer layers. Dichloromethane, similarly to water, formed microdroplets on the surface of the aluminum. In the case of using PET and PETP as supports, the formation of droplets on the substrate surface during drying was not observed so that islands of quasi-agglomerated nanowires are deposited. Due to the high difference in polarity between PVP and hexane, the forces between the PVP molecules adsorbed onto nanowires are much higher than those existing between the molecules of hexane and PVP, so that the wires are strongly agglomerated prior to settling regardless of the nature of the substrate used.
From the cases studied, the most appropriate method for silver nanowires layer deposition is by using of inks with ethanol as a dispersion medium and oxygen plasma treated PET substrates. However, even in this case, it can be observed a fairly high density of nanowires which are electrically unconnected to the mass of nanowires.
Objective 6 (2016): Optimization of Ag nanowires-based flexible, transparent and conducting electrodes to increase diffuse transmittance / resistance ratio
Optimizing of Ag nanowires-based flexible, transparent and conducting electrodes to increase the transmittance/resistance ratio (T/R) can be achieved in three ways: (i) Increasing the transmittance by reducing the density of nanowires; (ii) Lowering the resistance by decreasing the number of contacts between wires; (iii) Lowering the resistance by welding of wires with the decrease of contact resistance.
The results of the previous stages indicated that reduction of the density of nanowires leads to the improvement of transmittance but also to the rise of surface resistance due to the formation of clusters of silver nanowires shaped as "islands" which are electrically unbound or weakly bound to each other. To reduce the number of these "islands" composed of silver nanowires, a first measure taken is to increase the aspect ratio of the nanowires by increasing their average length. Thus, we synthesized silver nanowires with high aspect ratios. By XRD and SEM characterization it was observed that the use of PVP with a high degree of polymerization (Mw 1,300,000) leads to nanowires with aspect ratios higher than 100 in the case of using AgCl for the heterogeneous nucleation process.

SEM images of AgNWs with high aspect ratio.
Transparent, conductive electrodes have been obtained by Meyer rod, using an intermediate layer of polymethylmethacrilate to improve adhesion of nanowires to the substrate. Samples have been obtained by depositing 1, 2 and respectively 3 successive layers of AgNWs followed by drying at 150°C for 20 minutes. The surface resistance has been measured and the values before and after thermal treatment are given bellow.
Surface resistance of PET/PMMA/AgNW before thermal treatment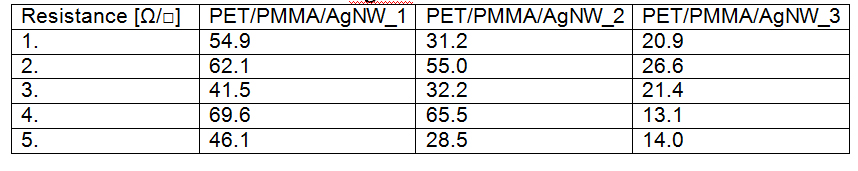
Surface resistance of PET/PMMA/AgNW after thermal treatment at 150°C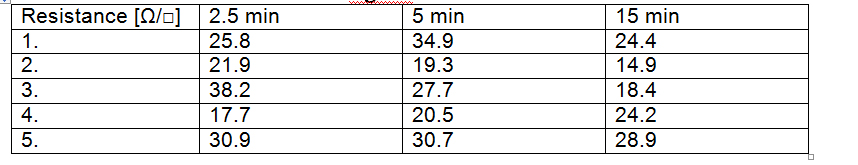
To study the effect of temperature on welding of the nanowires and to determine the optimum thermal treatment time, the electrical resistance variation was monitored during repeated heating and cooling cycles and after high temperature mechanical pressing.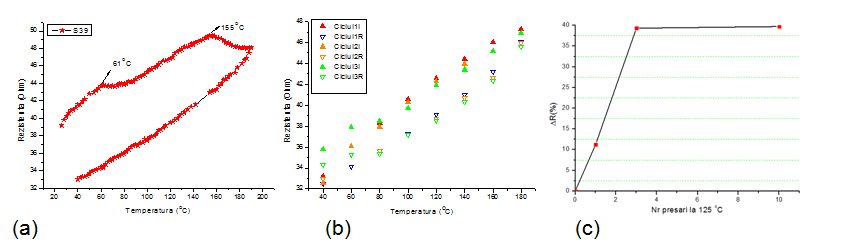
Variation of resistance with temperature for one heating-cooling cycle (a) and for subsequent 3 cycles (b). Variation of resistance with the number of pressing cycles at 125°C.
The results show that the resistance of AgNWs electrodes may be reduced by more than 20% by a simple heating of the substrate at a temperature of 180 ° C, followed by cooling. Subsequent heating and cooling cycles does not lead to significant change in the electrical resistance of the film. Hot pressing is the most efficient way to reduce the resistance, after 3 cycles of hot pressing a decrease by 35% has been obtained.
Objective 7 (2016): Deposition of a conducting polymer on previously manufactured electrodes
Studies have been carried out regarding the possibility of electrochemical deposition of polyaniline on the transparent, conductive AgNW-based electrodes. GC_AgNW electrodes have been obtained by drop casting on AgNW suspension on glassy carbon electrode. PET_AgNW electrodes have been obtained by Meyer rod deposition of AgNW suspension on PET substrate.
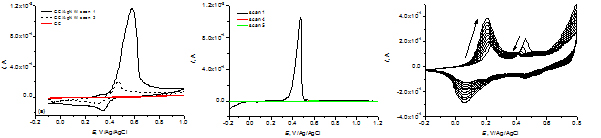
Cyclic voltammograms for GC_AgNW (a) and (b) electrodes in 0.5 M H2SO4 solution. Cyclic voltammograms for electrochemical deposition of polyaniline on GC_AgNW electrode (c).
Cyclic voltammograms for GC_AgNW and PET_AgNW electrodes show that oxidation of AgNW takes place easily, due to the existence of structural defects caused by mechanical stresses that occur between the five triangular prisms that compose a nanowire. The intensity of the oxidation peak is higher than that of the reduction peak and decreases considerably in the following cycles, indicating that AgNW dissolve during anodic polarization. Electrochemical deposition of polyaniline was possible only in case of GC_AgNW electrodes, where the substrate plays also a role in the electrodeposition.
As a result, in order to achieve transparent electrodes with conductive polymer, another conductive polymer was used, polyethylenedioxythiophene doped with polystyrene sulfonate (PEDOT: PSS) as a 0.8% aqueous suspension, applied by Meyer rod on the AgNW transparent conductive films. To improve the electrical properties for use in solar cells, platinum has been additionally deposited by galvanostatic pulse technique. The resulting electrodes, with and without platinum, were characterized for the reduction of triiodide to iodide (I3- + 2e- = 3 I-) which is the reaction generating the photocurrent in solar cells.
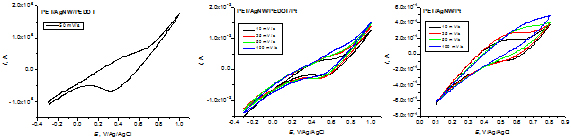
Cyclic voltammograms of transparent electrodes with conductive polymer and platinum in 0.1 M LiI / 0.01 M I2 in acetonitrile.
By comparing reduction peak currents, the activity of the electrodes for the reduction reaction of triiodide increases in the order: PET/AgNW/Pt < PET/AgNW/PEDOT/Pt < PET/AgNW/PEDOT.
Compared to AgNW electrodes without conducting polymer, the values of the surface resistance significantly decrease, because the AgNW are embedded in the matrix of conducting polymer and the conductivity increases and is more uniform over the entire surface.
The deposition of platinum however, leads to an increase of the resistance and decrease of the diffuse transmittance.
Surface resistance of AgNWW transparent films with conductive polymer and platinum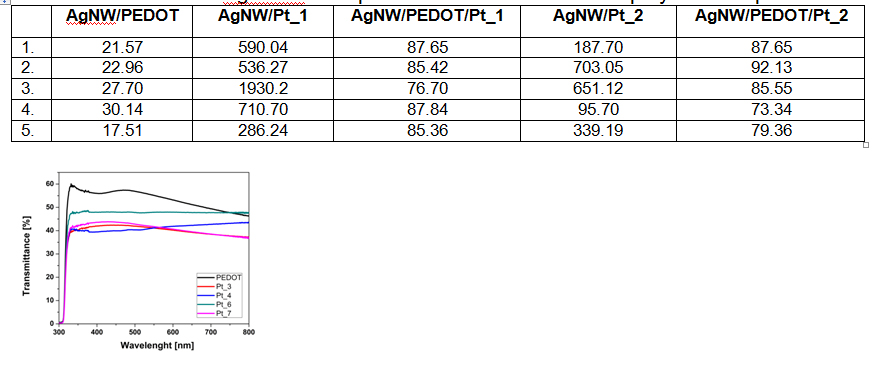
Objective 8 (2016): Construction of dye-sensitized solar cells using Ag nanowires-based transparent and conducting electrodes
The construction of dye sensitized solar cells (DSSC) implies following steps: (a) preparing the semiconductor paste based on TiO2; (ii) applying the semiconductor TiO2 paste on conductive fluorine doped tin oxide (FTO) glass followed by sintering at 450°C for 1 hour; (iii) treatment of TiO2 films with TiCl4¬ to improve electron injection and to reduce the recombination rate between electrolyte and semiconductor, followed by adsorption of dye based on ruthenium (N749 black dye) and (iv) assembly of solar cell. For the assembly of the solar cell, FTO/TiO2/TiCl4/N749 electrodes were used as anode and PEDOT/AgNW electrodes as cathodes. The electrolyte solution was 0.1 M LiI, 0.03 M I2 and 0.5 M 4-tert-butylpyridine in acetonitrile.

Images of FTO/TiO2 (a) and FTO/TiO2/TiCl4/N749 (b,c) electrodes and image of a solar cell prepared for testing (d).
The solar cells were tested using a solar simulator with irradiating power of 100 mW/cm2 and the corresponding i-V curves have been recorded with a Keithley 2450 digital multimeter. The results have been compared to a FTO/Pt counterelectrode, obtained by thermal dopisition of Pt on FTO.
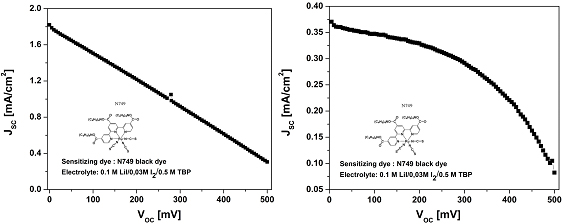
Characteristic I-V curves of solar cells constructed using PET/AgNW/PEDOT (a) and FTO/Pt (b) counterelectrodes.
For the use of PET/AgNW/PEDOT counterelectrode the short circuit current is about 1.8 mA/cm2 which is about 3 times higher than the corresponding value obtained for FTO/Pt counterelectrode. In conclusion, the use of transparent AgNW electrodes with conducting polymer leads to the increase of the performance of the solar cell.
Results – Publications
Patent application
1. R. Bănică, A. Kellenberger, D. Ursu, L. Cseh, P. Linul, N. Vaszilcsin, Procedeu de sinteză a nanofirelor de argint acoperite cu nanoparticule metalice cu punct de topire scăzut.
ISI Publications
1. R. Bănică, D. Ursu, T. Nyari, A. Kellenberger*, Solvothermal growth of pre-synthesized silver nanowires, Material Letters – under review
2. R. Bănică, D. Ursu, T. Nyari, A. Kellenberger*, Polyol synthesis of silver nanowires in the presence of silver chloride, Optoelectronics and Advanced Materials – Rapid Communication – under review
3. R. Bănică, D. Ursu*, P. Svera, C. Sarvaş, S.F. Rus, S. Novaconi, A. Kellenberger, A.V. Racu, T. Nyari, N. Vaszilcsin, Electrical properties optimization of silver nanowires supported on polyethylene terephtalate, Particulate Science and Technology, 34 (2016) 217-222, http://dx.doi.org/10.1080/02726351.2015.1066473
Non - ISI Publications
4. D. Ursu, R. Bănică, N. Vaszilcsin*, Photovoltaic performance of (Al, Mg)-doped CuCrO2 for p-type dye-senzitized solar cells application, Nanoscience and Nanotechnology 6 (2016) 71-76, DOI: 10.5923/c.nn.201601.14
Papers presented at international conferences:
1. R. Bănică, R. Băieş, R. Bucur, C. Locovei, A. Kellenberger, T. Nyari*, Study of liquid phase synthesis of silver nanowires for solar cell applications, 3rd European Energy Conference E2C, 27-30 octombrie, 2013, Budapesta, Ungaria
2. R. Bănică*, R. Băieş, D. Ursu, M. Poienar, T. Nyari, Silver nanowires synthesis in the PVP-silver chloride system, International Conference Ecoimpuls 2013 – Environmental Research and Technology, 7-8 noiembrie, 2013, Timişoara, România
3. R. Bănică*, C. Sarvaş, S.F. Rus, S. Novaconi, A. Kellenberger, T. Nyari, Optimization of the electrical and mechanical properties of transparent electrodes based on silver nanowires supported on polyethylene terephtalate, 7th International Symposium on Flexible Organic Electronics ISFOE 14, 7-10 iulie, 2014, Thessaloniki, Grecia
4. R. Bănică, C. Sarvaş, S.F. Rus, S. Novaconi, A. Kellenberger, T. Nyari, D. Ursu*, Manufacture of ultrathin transparent electrodes based on silver nanowires with applications to three-dimensional solar cells, 7th International Symposium on Flexible Organic Electronics ISFOE 14, 7-10 iulie, 2014, Thessaloniki, Grecia
5. L. Cseh*, C. Locovei, O. Marinica, A. Kellenberger, T. Nyari, D. Ursu, R. Bănică, Synthesis and characterization of indium nanoparticles as precursor for solar cells, New trends and strategies in the chemistry of advanced materials with relevance in biological systems, technique and environmental protection, 5-6 iunie, 2014, Timişoara, România
6. R. Bănică*, C. Moşoarcă, P. Linul, T. Nyari, N. Vaszilcsin, Indium decorated silver nanowires, 21st International Symposium on Analytical and Environmental Problems, 28 septembrie, 2015, Szeged, Ungaria.
7. D. Ursu*, R. Bănică, N. Vaszilcsin, Photovoltaic performance of (Al, Mg)-doped CuCrO2 for p-type dye senzitized solar cells application, Applied Nanotechnology and Nanoscience Conference, 5-7 noiembrie, 2015, Paris, Franţa
8. P. Linul*, R. Bănică, D. Ursu, C. Moşoarcă, P. Svera, N. Vaszilcsin, Optimization of transparent electrodes based on silver nanowires, TIM 15-16 Physics Conference, 26-28 mai, 2016, Timişoara, România
PhD thesis
1. Linul Petrică Andrei, Fabrication of transparent and conducting electrodes based on silver nanowires.
Dissertation / diploma works:
1. Linul Petrică Andrei – Optimization of silver nanowires synthesis and evaluation of behavior of transparent conducting electrodes during thermal treatment by heating-cooling cycles, 2016, University Politehnica Timisoara.
1. Claudia Sarvas – Deposition of thin films based on silver nanowires on transparent substrates, with applications in solar cells, 2014, University Politehnica Timisoara.
2. Arhip Liliana – Silver nanowires to improve the efficiency of solar panels, 2014, University Politehnica Timisoara.


